The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
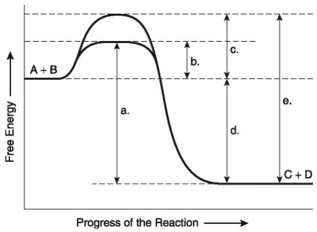
-Assume that the reaction in the figure above has a ΔG of -5.6 kcal/mol. Which of the following would be true?
Definitions:
Negative Emotion
Unpleasant or adverse feelings triggered by the perception of undesirable situations, events, or interactions.
Dimension
A measurable extent of some kind, such as length, width, height, or in broader contexts, an aspect or feature of a situation, problem, or thing.
Mood Congruency
The phenomenon where an individual's current mood influences their thoughts, behaviors, and perceptions in a way that is consistent with the mood.
Example
An instance or situation that is representative of a group or set of things, often used to illustrate a point or explain a concept.
Q10: Two plants are crossed, resulting in offspring
Q16: In the above diagram, the structure labelled
Q25: The phosphate transport system in bacteria imports
Q34: The figure above shows the structures of
Q42: Zinc, an essential trace element for most
Q55: Suppose a biologist can separate one of
Q59: When we see chiasmata under a microscope,
Q71: In the figure above, mitosis is represented
Q71: Which bond is closest to the amino
Q116: Why is glycolysis considered to be one