THE NEXT QUESTIONS ARE BASED ON THE FOLLOWING INFORMATION:
The results of a two-factor ANOVA without replication are displayed below:
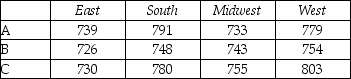
ANOVA: Two-Factor Without Replication
SUMMARY
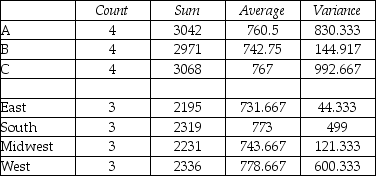
ANOVA
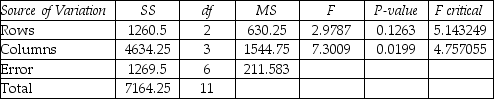
-Is there sufficient evidence to reject H0 that the average between programs A,B,and C regions is the same?
Definitions:
ΔG°
The standard Gibbs free energy change, a thermodynamic property indicating the spontaneity of a reaction at standard conditions.
Exothermic Reaction
A chemical reaction that releases energy in the form of heat, resulting in a lower energy state for the products compared to the reactants.
Stronger Bonds
Bonds characterized by high bond energy, indicating a stronger force of attraction between atoms.
Standard Gibbs Free Energy
A thermodynamic property used to predict the direction of chemical reactions and the extent to which reactions proceed.
Q41: The year _ has the forecast value
Q45: Find the coefficient of determination of this
Q69: Three predictor variables are being considered for
Q103: If for a particular data set,the skewness
Q112: Autoregressive forecasting models assume that values in
Q116: What is the seasonal index for quarter
Q122: The range of the values of the
Q125: Which of the following weeks has a
Q139: When the errors in a regression model
Q213: Data were collected regarding the amount of