An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
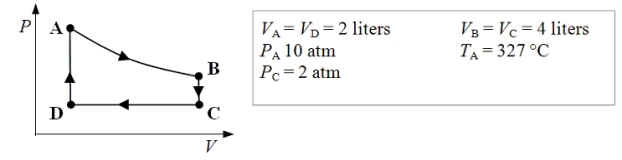
-How much work is done on the gas as it is compressed isobarically from state C to state D?
Definitions:
Q2: Some of the lowest pitches attainable on
Q15: Compared to other types of porn,femme porn
Q28: A sample of a monatomic ideal gas
Q30: A ray of light passes from air
Q37: Photons of what minimum frequency are required
Q54: What is the peak voltage?<br>A)10 V<br>B)60 V<br>C)120
Q56: A conducting loop has an area of
Q65: What is the total pressure at point
Q77: All of the following are "psychological or
Q87: Three 15-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6393/.jpg" alt="Three 15- and two