Beneath the moveable piston in the drawing,2.25 moles of a monatomic ideal gas is enclosed at 314 K.The initial volume of the gas is 3.0 m³.The gas is compressed isothermally to a final volume of 1.0 m³.How much heat is removed from the gas during this process? 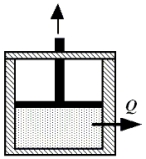
Definitions:
Trust
The firm belief in the reliability, truth, ability, or strength of someone or something, fundamental for building and maintaining relationships in social and professional contexts.
Accurately
Accurately refers to the quality of being precise, correct, or exact in the execution or representation of something.
Integrative Agreements
Refers to negotiation outcomes that involve cooperation between parties to achieve mutually beneficial results.
Sufficient Information
The minimum amount of data required to make an informed decision or conclusion.
Q1: Many prostitutes consider sex they are paid
Q6: A convex mirror with a focal length
Q16: Complete the following statement: Bernoulli's principle is
Q33: How much force does the atmosphere exert
Q41: What is the wavelength of the first
Q51: A transformer has 450 turns in its
Q55: When two capacitors are connected in series,the
Q70: Which of the following is a finding
Q72: Which of the following is referred to
Q110: Which of the following is the most