An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
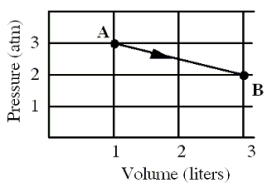
-Suppose that the same gas is originally in state A as described above,but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
Definitions:
Degrees
Degrees are academic qualifications awarded by colleges or universities, representing a level of education or expertise achieved in a specified field of study.
Varieties
Different types, categories, or forms of a specific thing, emphasizing diversity within a group or category.
Moral Panic
A widespread feeling of fear, often irrational, about a potential threat to societal norms and values, usually fueled by media and political discourse.
Crime Prevention
The set of practices, strategies, and actions aimed at stopping crimes from occurring.
Q15: An isobaric process is represented on a
Q23: Many colleges have developed sexual harassment policies
Q26: According to a study,Farley,2003,what percentage of streetwalkers
Q29: Assume that S has been closed for
Q32: Enclosed beneath the moveable piston in the
Q39: What is the approximate average power dissipated
Q40: A bubble with a volume of 1.0
Q56: A scuba diver shines a flashlight from
Q69: What is the magnitude of v?<br>A)1.5 m/s<br>B)4.6
Q79: During the Middle Ages,gay men<br>A)had higher status