In a reversible heat engine,one mole of an ideal gas is carried through a circular cycle beginning and ending at point A as shown in the figure.Which one of the following statements concerning this system is false? 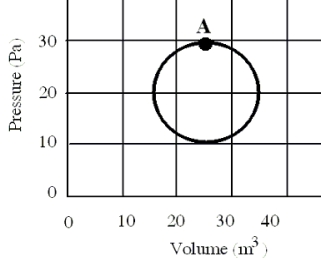
Definitions:
Bourdieu
A French sociologist, anthropologist, and philosopher known for his work on habitus, cultural capital, and social fields.
Deadnaming
The act of using the birth or previous name of a transgender or non-binary person without their consent, often considered disrespectful and invalidating.
Transgender
Those who deviate from the binary (that is, male or female) system of gender.
Marginalize
To relegate or confine to a lower or outer edge of society, often limiting access to resources and opportunities for those deemed outside the mainstream.
Q2: How much heat is added to the
Q4: Which law,principle,or equation can be used to
Q8: A guitar string produces 4 beats/s when
Q20: According to a study,Farley,2003,what percentage of streetwalkers
Q25: When plucked,a 0.62-m guitar string produces a
Q27: What is the pressure of the gas
Q34: An X-ray generator produces photons with energy
Q38: One study found that the most common
Q97: Sexual harassment is solely a behavior of
Q101: In the context of motivations for rape,the