An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
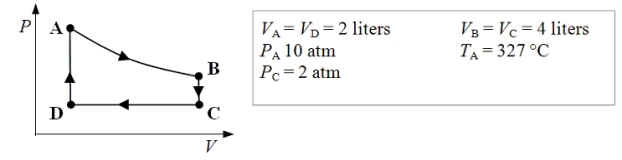
-What is the net amount of work done after one complete cycle?
Definitions:
Equivalent Units
A method used in process costing that converts partially completed units into a number of fully completed units.
Direct Materials Costs
The cost of raw materials that are directly used in the manufacture of a product.
FIFO Method
First-In, First-Out, an inventory valuation method where the first items purchased are the first ones to be removed from inventory.
Units Started and Completed
This term refers to items or products that begin and finish production within a specific accounting period, in the context of manufacturing or process accounting.
Q11: A 4.00-m long string,clamped at both ends,vibrates
Q16: Sound waves are emitted from two speakers.Which
Q23: Complete the following statement: The transfer of
Q25: When plucked,a 0.62-m guitar string produces a
Q27: In the figure,Joel is using a device
Q27: A tank contains 135 moles of the
Q31: How much heat is extracted from the
Q34: Determine the speed of the waves on
Q43: If the area of contact at the
Q88: Two wires,A and B,and a variable resistor,R,are