How many S 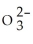 ions are contained in 99.6 mg of Na2SO3? The molar mass of Na2SO3 is 126.05 g mol-1.
ions are contained in 99.6 mg of Na2SO3? The molar mass of Na2SO3 is 126.05 g mol-1.
Definitions:
Q28: Which element has the chemical symbol S?<br>A)selenium<br>B)silicon<br>C)sulfur<br>D)scandium
Q40: Calculate the molar mass of H<sub>2</sub>CO<sub>3</sub>.<br>A)62.03 g
Q42: Why are clinical viral diseases more challenging
Q48: Determine the name for Cl<sub>2</sub>O.<br>A)chlorine oxide<br>B)dichlorine monoxide<br>C)chlorine(I)oxide<br>D)chlorine(II)oxide<br>E)chlorate
Q59: At what temperature does argon have a
Q70: A fishing boat accidentally spills 3.0 barrels
Q139: How many xenon atoms are contained in
Q154: Calculate the molecular weight of a gas
Q161: Determine the number of protons, neutrons, and
Q209: What is the concentration of NO<sub>3</sub><sup>-</sup> ions