Related Questions
Q1: What is the structure of the covalent
Q12: What element is undergoing oxidation (if any)in
Q25: Use Lewis theory to determine the chemical
Q38: What are the required coefficients to properly
Q47: Place the following types of electromagnetic radiation
Q56: Which of the following gases has the
Q93: Two solutions, initially at 24.69 °C, are
Q115: Calculate the energy of an electron in
Q145: Calculate the temperature, in K, of 2.20
Q161: The compound Cu(I<sub> </sub>O<sub>3</sub> )<sub>2</sub>,is named<br>A)copper iodate(II).<br>B)copper(I)iodate.<br>C)copper(I)iodate(II).<br>D)copper(II)iodate.
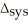 U = -95 J?
U = -95 J?