Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 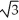 .
.
Definitions:
Cost Of Goods Sold
The direct costs attributable to the production of the goods sold by a company. This includes both the materials and labor costs.
General/Administrative Expenses
Overhead or operational costs not directly tied to producing goods or services, such as salaries of administrative staff.
Full-Capacity Level
The maximum level of output that a company can sustain over a period of time without incurring additional costs.
Balance Sheet
A financial statement showing a company's assets, liabilities, and shareholders' equity at a specific point in time.
Q4: Given the following proposed mechanism, predict the
Q5: Give the approximate bond angle for a
Q23: The hybrid orbital set used by the
Q28: The condensed electron configuration of krypton, element
Q56: Use the periodic table to predict the
Q74: Which of the following is the characteristic
Q76: Ethanol (C<sub>2</sub>H<sub>5</sub>OH)melts at -114 °C. The enthalpy
Q100: For the following reaction, what is Δn
Q109: Choose the aqueous solution with the highest
Q144: Define the frequency factor.