Express the equilibrium constant for the following reaction: 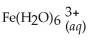 + 6CN-(aq) ⇌
+ 6CN-(aq) ⇌ 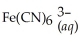 + 6H2O(l)
+ 6H2O(l)
Definitions:
Interest Expense
The cost incurred by an entity for borrowed funds, typically reported as a line item in the income statement.
Payout Ratio
The proportion of earnings paid out as dividends to shareholders, typically expressed as a percentage.
Dividend Payout Ratio
A financial measurement that calculates the percentage of net income distributed to shareholders in the form of dividends.
Retention Ratio
A financial metric indicating the percentage of a company's income retained and reinvested after dividends are paid to shareholders.
Q6: If an equal number of moles of
Q59: At a certain temperature the equilibrium constant,
Q60: What is the pH of a solution
Q67: The isomerization of methylisonitrile to acetonitrile CH<sub>3</sub>NC(g)→
Q72: What is the name of the reaction
Q96: The molar solubility of ZnS is 1.6
Q105: Describe the information necessary (and why)to choose
Q128: Calculate the activation energy, E<sub>a</sub>, for a
Q141: Determine the molar solubility of MgCO<sub>3 </sub>in
Q143: Identify acid, base, conjugate acid, and conjugate