Phosphorus trichloride and phosphorus pentachloride equilibrate in the presence of molecular chlorine according to the following reaction: 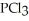 (g) +
(g) + 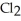 (g) →
(g) → 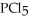 (g) An equilibrium mixture at 450 K contains
(g) An equilibrium mixture at 450 K contains 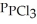 = 0.124 bar,
= 0.124 bar, 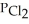 = 0.157 bar, and
= 0.157 bar, and 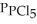 = 1.30 bar. What is the value of Kp at this temperature?
= 1.30 bar. What is the value of Kp at this temperature?
Definitions:
Responsibility
The state or fact of having a duty to deal with something or of having control over someone, often implying accountability for one's actions.
Strategic Planning
The process of defining an organization's strategy or direction and making decisions on allocating its resources to pursue this strategy.
Organization's Success
The achievement of the specific goals and objectives set by an organization, measured by various indicators such as profitability, market share, or social impact.
Chief Executive Officer
The Chief Executive Officer (CEO) is the highest-ranking executive in a company or organization, responsible for making major corporate decisions, managing the overall operations, and acting as the main point of communication between the board of directors and the corporate operations.
Q7: CH<sub>3</sub>OH<br>A)dispersion forces<br>B)dipole-dipole forces<br>C)ion-dipole forces<br>D)hydrogen bonding<br>E)ionic bond<br>F)H<sub>2</sub> +
Q8: k<br>A)activation energy<br>B)half-life<br>C)reaction order<br>D)rate constant<br>E)frequency factor
Q10: At 20 °C, a 2.32 mol L<sup>-1</sup>
Q29: The acid-dissociation constant at 25.0 °C for
Q38: Consider the following reaction at equilibrium. What
Q56: Determine the molality of a solution prepared
Q57: A 100.0 mL sample of 0.10 mol
Q100: The boiling point elevation of an aqueous
Q116: The boiling point of water is _.<br>A)0
Q144: Define the frequency factor.