What is the hydronium ion concentration of a 0.150 mol L-1 hypochlorous acid solution with Ka = 3.5 × 10-8? The equation for the dissociation of hypochlorous acid is below: 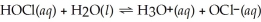
Definitions:
Neural Activity
The electrical and chemical processes that occur within the brain and nervous system, facilitating functions such as thought, movement, and sensation.
Pain And Anxiety
The interrelation of discomfort and worry, where pain can cause anxiety, and anxiety can increase the perception of pain.
Endorphins
Neurochemicals produced within the body that act as natural painkillers and mood elevators, often credited with producing feelings of pleasure and euphoria.
Neurotransmitters
Substances that carry signals from one neuron to another across a synapse in the nervous system.
Q5: Lewis acid<br>A)proton donor<br>B)electron pair acceptor<br>C)proton acceptor<br>D)electron pair
Q7: Which of the following statements is true
Q10: In the Haber process, ammonia is synthesized
Q22: What is "free" energy? Give a fictitious
Q46: Given that E°<sub>red</sub> = -0.40 V for
Q57: For the reaction: N<sub>2</sub>(g)+ 2O<sub>2</sub>(g)⇌ 2NO<sub>2</sub>(g), K<sub>c</sub>
Q64: A solution is prepared by adding 30.00
Q69: Determine the equilibrium constant for the following
Q120: How many grams of chromium metal are
Q120: Which of the following bases is the