Determine the ammonia concentration of an aqueous solution that has a pH of 11.00. The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 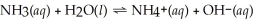
Definitions:
Positive Outcomes
The beneficial or desired results of an action or set of actions, often used in the context of goals and objectives.
Behavior Control
The manipulation of an individual's actions through regulations, rules, or other managerial strategies.
Position Power
The authority and influence a person possesses within an organization, derived from their job or official position.
Personal Power
The influence or authority a person has over others, derived from their skills, qualities, or personal attributes, rather than their formal position.
Q13: A galvanic cell consists of one half-cell
Q17: A reaction with an activation energy of
Q18: Consider a reaction that has a negative
Q31: What is the pH of the resulting
Q38: Describe what changes occur in the atomic
Q39: Q > K<br>A)reaction will favour formation of
Q59: A solution of methanol and water has
Q92: Express the equilibrium constant for the following
Q92: The first-order decay of radon has a
Q106: The first-order reaction, 2N<sub>2</sub>O(g)→ 2N<sub>2</sub>(g)+ O<sub>2</sub>(g), has