What is the pH of a 0.100 mol L-1 NH3 solution that has Kb = 1.8 × 10-5? The equation for the dissociation of NH3 is below: 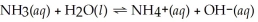
Definitions:
Aptitude Test
A test that predicts future performance in a particular setting or on a specific task.
Standardized Test
A test administered and scored in a consistent manner, used to measure a participant's knowledge or skills in a specific area.
Reliable Test
A measure that consistently produces the same results under the same conditions, indicating the test's stability and consistency over time.
Bell Curve
A graphical depiction of a normal distribution, showing that data near the mean are more frequent in occurrence than data far from the mean.
Q12: The rate of disappearance of HBr in
Q16: Which particle has the lowest penetrating power?<br>A)alpha
Q55: Define half-life.
Q63: Which rate law is termolecular?<br>A)rate = k[A][B]<sup>2</sup><br>B)rate
Q66: Determine the pOH of a 0.741 mol
Q67: Express the equilibrium constant for the following
Q76: Express the equilibrium constant for the following
Q91: Determine the pH of a 0.116 mol
Q97: Q > K<br>A)E°<sub>cell</sub> < 0<br>B)E<sub>cell </sub>< 0<br>C)E°<sub>cell</sub>
Q128: Calculate the activation energy, E<sub>a</sub>, for a