If the pH of 1 liter of a 1.0 M carbonate buffer is 7.0,what is actual number of moles of H2CO3 and HCO3-? (pK = 6.37) 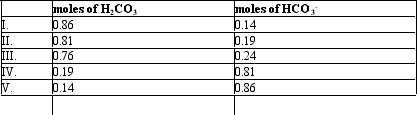
Definitions:
Dividend
A payout of a portion of a company's earnings to its shareholders, typically in the form of cash or stock.
Sole Proprietorship
Sole Proprietorship is a business structure owned and operated by one individual, where there is no legal distinction between the owner and the business.
Legal Entities
Entities recognized by law that have rights and responsibilities, such as corporations, partnerships, and sole proprietorships.
Accounting Entities
Organizations, businesses, or units for which separate financial statements are prepared, distinct from any other economic activities.
Q5: The peptide bond<br>A) is formed by elimination
Q9: Which of the following is true?<br>A) The
Q16: Nitrogenase uses which of the following cofactors?<br>A)
Q18: Glycogen breakdown proceeds from the nonreducing ends.
Q21: Which of the following is not associated
Q27: Glucose-6-phosphatase activity is found associated with the
Q33: Exhibit 19A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1882/.jpg" alt="Exhibit 19A
Q49: Which of the following proteins are produced
Q49: The standard state usually used in biochemistry
Q82: In a Fischer projection,which chiral carbon determines