How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 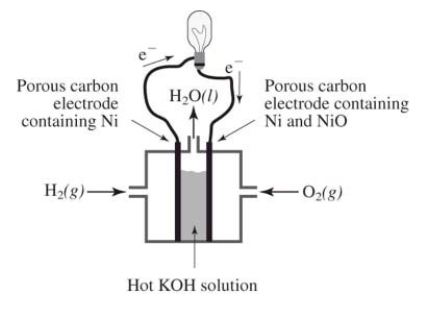 I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
II) Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen
III) In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction
IV) It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell
Definitions:
Nondirective Interview
An interview in which the applicant is allowed the maximum amount of freedom in determining the course of the discussion, while the interviewer carefully refrains from influencing the applicant’s remarks.
Maximum Freedom
The highest level of autonomy granted to individuals or entities, often within a legal or organizational context.
Eligible To Be Bonded
A status indicating that an individual or company can be insured against losses caused by dishonesty, fraud, or failure to perform.
Application Form
A document that an individual fills out to apply for something, such as a job, loan, or program, detailing personal, educational, and professional information.
Q6: Which of the following is NOT likely
Q6: DNA has four different nitrogen-containing bases.Which of
Q13: Recall that McCullough et al. (2003,2005)examined stability
Q14: The quantity 8.7 × 10<sup>5</sup> g expressed
Q26: Which of these are considered to be
Q31: A structure-activity-relationship study<br>A)considers the relationship of many
Q39: Small changes in _ protein structure may
Q40: When it reaches its largest size,the ozone
Q46: Approximately what percentage of the electrical energy
Q77: Which best symbolizes the hydrogen bonding between