Multiple Choice
Use the data given below to construct a Born-Haber cycle to determine the second ionization energy of Ca. DH°(kJ)
Ca(s) → Ca(g) 193
Ca(g) → Ca⁺(g) + e⁻ 590.
2 O(g) → O2(g) -498
O(g) + e⁻ → O⁻(g) -141
O⁻(g) + e⁻ → O2⁻(g) 878
Ca(s) + 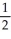 O2(g) → CaO(s) -635
O2(g) → CaO(s) -635
Ca2⁺(g) + O2⁻(g) → CaO(s) -3414
Definitions:
Related Questions
Q10: Why does the size of the transition
Q10: The atomic number of an atom of
Q46: What is the energy of light associated
Q57: Draw the best Lewis structure for BrO<sub>4</sub>⁻
Q77: What species is represented by the following
Q80: What is the concentration of an AlCl<sub>3</sub>
Q105: How many moles are in 2.16 ×
Q110: Calculate the mass (in mg)of 4.82 ×
Q128: Determine the empirical formula for a compound
Q189: Which of the following is an ionic