Lithium and nitrogen react to produce lithium nitride: 6Li(s) + 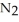 (g) →
(g) → 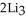 N(s)
N(s)
How many moles of 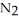 are needed to react with 0.450 mol of lithium?
are needed to react with 0.450 mol of lithium?
Definitions:
Logarithmic Approach
A method involving the use of logarithms, often employed in mathematical modeling, data analysis, and algorithm design to simplify complex problems.
Learning Curve
represents the rate at which a new skill is learned or proficiency is gained, often graphed as performance improvements over time with increased experience.
Logarithmic Approach
Utilizing logarithms, a mathematical concept related to exponents and power functions, to solve problems or understand data trends.
Q8: What is the enthalpy change (in kJ)of
Q15: Determine the electron geometry,molecular geometry and polarity
Q24: How much energy is evolved during the
Q52: A 1.44-g sample of an unknown pure
Q54: The total pressure of a gas mixture
Q89: Calculate the molar mass of Ba(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>.<br>A) 128.53
Q103: Identify the oxidation state of H in
Q105: How many electrons are in the ion,S<sup>2-</sup>?<br>A)
Q124: Which of the following elements is a
Q127: What volume will 0.780 moles of Xe