Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + 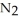 (g) →
(g) → 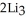 N(s)
N(s)
In a particular experiment,1.00-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
Definitions:
Specific Identification
An inventory costing method where each item of inventory is individually valued and tracked.
Cost of Goods Sold
Represents the direct costs attributable to the production of the goods sold by a company.
Average Cost Formula
A method used in accounting to calculate the cost of goods sold and ending inventory by averaging the cost of goods available for sale.
Perpetual Inventory System
An inventory management system that continuously updates the quantity and value of inventory on hand after each transaction.
Q2: Define ionization energy.
Q12: Calcium hydride ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6105/.jpg" alt="Calcium hydride
Q20: Use molecular orbital theory to determine whether
Q22: Give the hybridization for the Br in
Q29: What element is undergoing oxidation (if any)in
Q40: Balance the following equation.<br>_ C<sub>10</sub>H<sub>12</sub> + _
Q54: Give the electron geometry (eg),molecular geometry (mg),and
Q67: A 0.334 g sample of an unknown
Q106: The specific heat capacity of solid copper
Q117: Astatine belongs to the _ group of