Lithium and nitrogen react to produce lithium nitride: 6Li(s) + 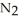 (g) →
(g) → 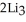 N(s)
N(s)
How many moles of lithium nitride are produced when 0.550 mol of lithium react in this fashion?
Definitions:
Beeping Sound
A type of auditory signal or notification often used by devices to attract attention or indicate an alert.
Electric Shock
A sudden discharge of electricity through a part of the body causing injury or neurological disturbance.
Extinction
In psychology, the gradual weakening and disappearance of a conditioned response, typically as a result of the conditioned stimulus being presented without reinforcement.
Classical Conditioning
A learning process in which a neutral stimulus comes to elicit a response after being paired with a stimulus that naturally elicits that response.
Q7: Which of the following reactions is associated
Q19: A molecule containing a central atom with
Q47: Determine the theoretical yield of H<sub>2</sub>S (in
Q57: Draw the best Lewis structure for BrO<sub>4</sub>⁻
Q61: If the density of ethanol,C<sub>2</sub>H<sub>5</sub>OH,is 0.789 g/mL.How
Q86: Identify the compound with ionic bonds.<br>A) Ne<br>B)
Q120: Which of the following elements is a
Q131: How many lone pairs are on the
Q144: It takes 11.2 kJ of energy to
Q147: If 100.mL of 0.200 M Na<sub>2</sub>SO<sub>4</sub> is