Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + 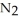 (g) →
(g) → 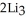 N(s)
N(s)
In a particular experiment,1.00-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
Definitions:
Age Groupings
Classification of data or people into groups based on their age.
Interest Revenue
Income earned from lending funds or investing in interest-bearing financial instruments.
Notes Receivable
Financial claims against others that are documented by formal legal instruments indicating a right to receive payments.
Journal Entry
A record of the financial transactions in the accounting books of a business, showing the accounts and amounts debited and credited.
Q7: Which of the following reactions is associated
Q10: How many of the following molecules have
Q21: Give the hybridization for the O in
Q37: What reagent could NOT be used to
Q50: Identify the compound with the highest percent
Q59: Draw the Lewis structure for the molecule
Q74: Which of the following statements about energy
Q97: When an aqueous solution of manganese (II)nitrate
Q127: Choose the best Lewis structure for NH<sub>4</sub>⁺.<br>A)
Q158: How many H<sup>+</sup> ions can the acid,H<sub>2</sub>SeO<sub>4</sub>