Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + 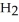 O(l) →
O(l) → 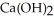 (s)
(s)
In a particular experiment,a 5.50-g sample of CaO is reacted with excess water and 6.77 g of 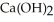 is recovered.What is the percent yield in this experiment?
is recovered.What is the percent yield in this experiment?
Definitions:
Equilibrium Price
The price in the market where the amount of products provided matches the amount of products wanted.
Demand
The quantity of a good or service that consumers are willing and able to purchase at various prices during a given period of time.
Supply
The overall quantity of a product or service accessible to buyers.
Inverse Supply Curve
A representation of the relationship between the price of a good and its supply, showing how quantity supplied decreases as price decreases, contrary to the typical direct supply relationship.
Q2: At 1 atm pressure,the heat of sublimation
Q6: When 10.00 moles of H<sub>2</sub>(g)reacts with 5.000
Q46: How many of the following elements can
Q69: When dissolved in water,RbOH behaves as<br>A) an
Q71: Determine the total volume of all gases
Q101: Which one of the following contains 35%
Q135: How many grams of XeF<sub>6</sub> are required
Q161: What is the charge on the Fe
Q173: Convert 1.75 atm to psi.<br>A) 52.4 psi<br>B)
Q181: Calculate the temperature,in K,of 2.20 moles of