Pure acetic acid ( 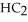
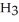
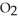 ) is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
) is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
Definitions:
Rigidity
The lack of flexibility or adaptability in processes, systems, or organizations that can hinder responsiveness and innovation.
Flexibility
The ability of a system, process, or entity to adapt to changes or demands without significant detriment or loss of functionality.
Consolidate Manufacturing
The process of centralizing or combining the production operations and facilities to improve efficiency and reduce costs.
Flexible Technology
Systems or equipment that can easily adapt to changes in production volume, design, or process, enhancing a company's ability to respond to market changes.
Q4: Consider the following balanced reaction.How many grams
Q13: Give the percent yield when 28.16 g
Q19: A 55.0-L steel tank at 20.0°C contains
Q26: A molecule containing a central atom with
Q94: Which of the following solutions will have
Q130: Describe the difference between complete ionic and
Q135: Draw the Lewis structure for NO<sub>2</sub>⁻ including
Q156: Identify sugar.<br>A) strong electrolyte, weak acid<br>B) weak
Q175: How many milliliters of a 9.0 M
Q179: To what volume will a sample of