Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 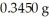 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
Definitions:
Seventeenth-Century
Refers to the period from 1601 to 1700 in the Gregorian calendar, marked by significant cultural, scientific, and political developments worldwide.
English Philosopher
A scholar specializing in philosophy from England who contributes to discussions and literature in the field of philosophy.
Aggression
A range of behaviors intended to harm or assert dominance over others, which can be physical, verbal, or nonverbal.
Inborn Features
Traits or characteristics present in an individual from birth, resulting from genetic inheritance.
Q7: What is the name for an aqueous
Q9: Consider the following reaction.How many moles of
Q14: Determine the concentration of a solution prepared
Q23: Choose the bond below that is LEAST
Q37: Propanol has a normal boiling point of
Q58: Which one of the following statements is
Q70: Give the number of valence electrons for
Q143: Calculate the work,w,gained or lost by the
Q155: Use Lewis theory to determine the chemical
Q161: Give the net ionic equation for the