Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 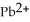 (aq) +
(aq) + 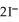 (aq) →
(aq) → 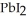 (s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many milliliters of 3.550 M HI(aq) must be added to a solution containing 0.500 mol of
(s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many milliliters of 3.550 M HI(aq) must be added to a solution containing 0.500 mol of 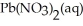 to completely precipitate the lead?
to completely precipitate the lead?
Definitions:
Alkyllithium
A class of organolithium compounds characterized by a direct bond between a lithium atom and a carbon atom in an alkyl group.
Acetic Acid
A simple carboxylic acid recognized as the main component of vinegar apart from water, with the formula CH3COOH.
2-Butanone
An organic compound, also known as methyl ethyl ketone, with the formula CH3COC2H5, used as a solvent.
Acid Chloride
A class of organic compounds derived from acids by replacing a hydroxyl group with a chlorine atom, often used as intermediates in synthesis.
Q3: Determine the electron geometry (eg)and molecular geometry
Q7: Choose the best Lewis structure for SF<sub>4</sub>.<br>A)
Q20: Use molecular orbital theory to determine whether
Q49: Given the equation C<sub>2</sub>H<sub>6</sub>(g)+ O<sub>2</sub>(g)→ CO<sub>2</sub>(g)+ H<sub>2</sub>O(g)(not
Q70: What is the molar mass of pentane
Q102: Define hypoxia.<br>A) oxygen starvation<br>B) increased oxygen concentration
Q111: Use the bond energies provided to estimate
Q114: The freezing point of water is<br>A) 32<sup>o</sup>F<br>B)
Q121: What is the strongest type of intermolecular
Q121: Place the following in order of increasing