Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: 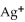 (aq) +
(aq) + 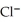 (aq) → AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.175 M
(aq) → AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.175 M 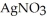 solution to completely precipitate the silver?
solution to completely precipitate the silver?
Definitions:
High-need Achievers
Individuals with a strong desire for success and accomplishment, often setting challenging goals and seeking feedback on their performance.
Achievable Goals
Objectives set with a realistic expectation of success, considering all resources and constraints, to ensure they can be attained.
Feedback on Performance
Input or assessments given to individuals about their work or performance, aimed at improvement.
Herzberg's Advice
Recommendations derived from Frederick Herzberg's Motivation-Hygiene Theory, focusing on improving job satisfaction to motivate employees.
Q16: Determine the hybridization about each interior atom
Q20: How many moles of SnCl<sub>2</sub> will be
Q56: How many grams of Li<sub>3</sub>N can be
Q59: Determine the vapor pressure (in mm Hg)of
Q98: Write the reaction illustrating ΔH°<sub>f</sub> of CaCO<sub>3</sub>
Q112: Determine the electron geometry (eg),molecular geometry (mg),and
Q117: Which of the following compounds is an
Q137: Give the number of valence electrons for
Q178: Using the graph below,determine the gas that
Q181: Calculate the temperature,in K,of 2.20 moles of