Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 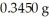 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
Definitions:
Classes of Common Stock
Different types of equity shares issued by a company, each with specific rights, privileges, or voting powers.
Voting Rights
The rights of shareholders to vote on company matters, typically exercised at shareholder meetings.
Dividends
Payments made by a corporation to its shareholder members, usually derived from the company's earnings.
Supernormal Growth
A period during which a company experiences significantly higher than average growth rates, often due to unique competitive advantages.
Q9: The combustion of titanium with oxygen produces
Q12: What is the chemical formula for strontium
Q26: What is the temperature of 144.32 g
Q43: Give the term for the temperature at
Q50: Using the following equation for the combustion
Q59: Draw the Lewis structure for the molecule
Q83: Draw the Lewis structure for CO<sub>3</sub><sup>2</sup>⁻.How many
Q101: Choose the best Lewis structure for NO<sub>3</sub>⁻.<br>A)
Q135: How many grams of XeF<sub>6</sub> are required
Q172: Determine the oxidation state of Ti in