What is the molarity of a NaOH solution if 39.1 mL of a 0.112 M 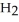
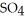 solution is required to neutralize a 25.0-mL sample of the NaOH solution?
solution is required to neutralize a 25.0-mL sample of the NaOH solution?
Definitions:
Self-Contradictory
A declaration or claim that contradicts itself or lacks logical consistency.
Conjunction
In logic and grammar, a connective word or phrase that joins together sentences, phrases, concepts, or elements of the same logical type.
Self-Contradictory
A statement or proposition that contradicts itself or is logically inconsistent.
Inconsistent
Describes a condition of being in conflict, having statements or beliefs that cannot all be true at the same time.
Q3: The normal boiling point for H<sub>2</sub>Se<sub> </sub>is
Q5: Which of the following would have a
Q8: Give the name for P Cl<sub>3</sub>.<br>A) phosphorus
Q66: Which of the following resonance structures for
Q74: What is the total pressure in a
Q78: Give the complete ionic equation for the
Q99: Give the units of specific heat capacity.<br>A)
Q130: Use the information provided to determine ΔH°<sub>rxn</sub>
Q145: Silver ions can be precipitated from aqueous
Q156: Identify sugar.<br>A) strong electrolyte, weak acid<br>B) weak