When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C.If the specific heat of the solution is 4.18 J/(g ∙ °C) ,calculate  for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g) 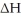 = ?
= ?
Definitions:
Aggressive Behavior
Actions or attitudes that are forceful, hostile, or attacking, often harmful to oneself or others.
High School Football
A team sport played by high school students in the United States, combining physical conditioning, strategy, and teamwork.
Anadrol
A synthetic anabolic steroid medication used primarily in the treatment of anemias caused by deficient red cell production, known by the generic name oxymetholone.
Dating
The social activity of engaging in planned meetings or outings with individuals to assess mutual compatibility as potential partners.
Q5: Give the coordination number for a body-centered
Q11: Identify the characteristics of a liquid.<br>A) indefinite
Q18: Of the following elements,which has the lowest
Q22: Draw the Lewis structure for the acetate
Q40: Draw the Lewis structure for the molecule
Q56: Consider the following compound.How many sigma and
Q73: Place the following substances in order of
Q78: Give the complete ionic equation for the
Q82: Parts per billion requires a multiplication factor
Q117: A sample of air from a home