How much heat is absorbed/released when 25.00 g of NH3(g) reacts in the presence of excess 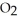 (g) to produce NO(g) and H2O(l) according to the following chemical equation?
(g) to produce NO(g) and H2O(l) according to the following chemical equation?
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l) ΔH° = 1168 kJ
Definitions:
Federal Funds Rate
The interest rate at which depository institutions trade federal funds (balances held at Federal Reserve Banks) with each other overnight.
Federal Reserve
The central banking system of the United States, responsible for monetary policy, regulation of financial institutions, and stability of the financial system.
Open-market Purchase
The buying of securities by a central bank from the market to inject liquidity and encourage economic growth.
Aggregate Demand
The complete need for goods and services across an economy at an established price level within a specific period.
Q8: Draw the best Lewis structure for ClO⁻
Q12: Calcium hydride ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6105/.jpg" alt="Calcium hydride
Q19: How many grams of AgNO<sub>3</sub> are needed
Q27: What volume (in mL)will a sample of
Q38: A student is preparing to perform a
Q41: Which of the following substances would you
Q51: When 1.50 g of Ba(s)is added to
Q52: Calculate the energy that is required to
Q112: How many milliliters of 0.200 M FeCl<sub>3</sub>
Q158: How many H<sup>+</sup> ions can the acid,H<sub>2</sub>SeO<sub>4</sub>