In the presence of excess oxygen,methane gas burns in a constant-pressure system to yield carbon dioxide and water: 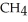 (g) +
(g) + 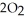 (g) →
(g) → 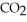 (g) +
(g) + 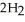 O(l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
O(l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
Definitions:
Risk
The probability of encountering harm or a loss in a given situation.
Health Psychology
A branch of psychology that focuses on how biological, psychological, and social factors affect health and illness.
Cardiac Rehabilitation
A customized program of exercise, education, and support to help patients recover after a heart attack, surgery, or other heart conditions.
Role
The function or part played by a person or thing in a particular situation or context.
Q4: Choose the situation below that would result
Q11: Which of the following compounds will be
Q17: Cesium has a radius of 272 pm
Q55: If the percent yield for the following
Q78: A 0.600 g sample containing Ag<sub>2</sub>O and
Q81: How many milliliters of ozone gas at
Q99: Give the units of specific heat capacity.<br>A)
Q161: An instrument used to measure blood pressure
Q173: What is the concentration of nitrate ions
Q184: Convert 1.50 atm to mm Hg.<br>A) 760