The fluorocarbon C2CL3F3 has a normal boiling point of 47.6°C.The specific heats of C2CL3F3(1) and C2CL3F3(g) are 0.91 J/gK and 0.67 J/gK,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 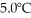 to the gas at 80.0°C is ________ kJ.
to the gas at 80.0°C is ________ kJ.
Definitions:
Purging-Type
A subtype of eating disorders characterized by behaviors such as self-induced vomiting or misuse of laxatives to prevent weight gain.
Preoccupation
A state of mind consumed by persistent thoughts or concerns, often distracting from other aspects of life.
Food
Any nutritious substance that people or animals eat or drink or that plants absorb in order to maintain life and growth.
Semistarvation Diet
A diet that provides insufficient caloric intake to maintain normal body functions, often leading to severe physical and mental health consequences.
Q5: Which of the following processes are endothermic?<br>A)
Q6: When 10.00 moles of H<sub>2</sub>(g)reacts with 5.000
Q8: Identify the type of solid for AgCl.<br>A)
Q22: According to the following reaction,how much energy
Q24: Determine the rate law and the value
Q25: How much energy is required to heat
Q91: In a liquid,the energy required to increase
Q115: What is △n for the following equation
Q139: All the following are state functions EXCEPT<br>A)
Q149: Given the following balanced equation,determine the rate