Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 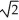 r.
r.
Definitions:
Internal Failure Cost
Costs incurred due to defects in goods or services before they are delivered to the customer, part of quality management expenses.
Quality Cost Report
A financial report detailing the costs associated with ensuring and maintaining the quality of products or services, including prevention, appraisal, and failure costs.
Appraisal Cost
Costs incurred to ensure that a product or service meets quality standards, including testing, inspections, and certifications.
Quality Cost Report
A financial report that details the costs associated with preventing, detecting, and dealing with defects in products.
Q4: Where does the energy absorbed during an
Q5: Which of the following would have a
Q40: Determine the value of K<sub>c</sub> for the
Q56: Express the equilibrium constant for the following
Q77: How many half-lives are required for the
Q92: Explain the difference between ΔH and DE.
Q121: When 5.00 mol of benzene is vaporized
Q135: Which of the following represents the integrated
Q153: Which of the following samples has the
Q174: How many liters of hydrogen gas can