Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 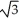 .
.
Definitions:
Dependent Variable
In an experiment or study, the variable being tested and measured, expected to change as a result of variations in the independent variable.
Direction
In the context of statistics, it refers to the nature of the relationship between variables, indicating whether it is positive or negative.
Correlations
Statistical measures that describe the extent to which two variables change together.
Q3: The isomerization reaction,CH<sub>3</sub>NC → CH<sub>3</sub>CN,is first order
Q17: Determine the vapor pressure of a solution
Q18: A 0.465 g sample of an unknown
Q20: When an X-ray beam with a wavelength
Q21: The two strands in DNA are held
Q55: Given three cylinders containing O<sub>2</sub> gas at
Q87: Define the frequency factor.
Q101: A solution containing less than the equilibrium
Q107: The boiling point of an aqueous 1.83
Q154: Why doesn't Dalton's Law of Partial Pressures