Multiple Choice
At 20°C,an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 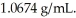 What is the molarity of ammonium chloride in the solution? The formula weight of NH4Cl is 53.50 g/mol.
What is the molarity of ammonium chloride in the solution? The formula weight of NH4Cl is 53.50 g/mol.
Definitions:
Related Questions
Q6: What function do enzymes serve?
Q9: Which one of these will diffuse the
Q36: What is △n for the following equation
Q38: Which Brønsted-Lowry acid is not considered to
Q70: Identify the weak diprotic acid.<br>A) HNO<sub>3</sub><br>B) H<sub>3</sub>PO<sub>4</sub><br>C)
Q82: Identify the base that is in Drano.<br>A)
Q92: What is the pH of a 0.50
Q93: A scuba diver experiences _ as she
Q96: Determine the value of K<sub>p</sub> for the
Q141: Give the major force between ethanol and