The Kp for the reaction below is 1.49 × 108 at 100.0°C: CO(g) + Cl2(g) → COCl2(g)
In an equilibrium mixture of the three gases,PCO = 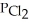 = 4.44 × 10-4 atm.The partial pressure of the product,phosgene (COCl2) ,is ________ atm.
= 4.44 × 10-4 atm.The partial pressure of the product,phosgene (COCl2) ,is ________ atm.
Definitions:
Net Income
Net income is the ultimate profit a company achieves after subtracting all taxes, expenses, and costs from its total income.
Commercial Substance
A situation where the future cash flows of a business are expected to change significantly as a result of a transaction.
Plant Assets
Long-term tangible assets used in the operation of a business that are not intended for sale.
Recognized Immediately
The principle of recording revenues or expenses in the accounting period when they occur, regardless of when cash is received or paid.
Q13: Calculate the pOH of a solution that
Q17: Can the K<sub>p</sub> and K<sub>c</sub> for a
Q23: Which of the following statements is TRUE?<br>A)
Q64: Calculate ΔS°<sub>rxn</sub> for the following reaction.The S°
Q70: Identify the weak diprotic acid.<br>A) HNO<sub>3</sub><br>B) H<sub>3</sub>PO<sub>4</sub><br>C)
Q72: What is the selenide ion concentration [Se<sup>2-</sup>]
Q74: Which rate law is termolecular?<br>A) rate =
Q86: _ is applied to roads in wintertime
Q130: A 25.0-mL sample of 0.150 M benzoic
Q133: Calculate P [NO]eq,if P [NOCl]<sub>eq</sub> = 0.33