Phosphorous Trichloride and Phosphorous Pentachloride Equilibrate in the Presence of Molecular
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: PCl3(g) + Cl2(g) → PCl5(g)
An equilibrium mixture at 450 K contains 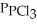 = 0.124 atm,
= 0.124 atm, 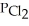 = 0.157 atm,and
= 0.157 atm,and 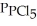 = 1.30 atm.
= 1.30 atm.
What is the value of Kp at this temperature?
Definitions:
Group Norms
Unwritten set of expectations for group members—what people ought to do; basic principle to follow in getting along with coworkers
Orientation Programs
Training sessions designed to familiarize new employees with the policies, procedures, and culture of an organization.
Being Civil
The act of being polite and respectful in interactions with others, demonstrating good manners and courtesy.
Basic Manners
Fundamental etiquettes or behaviors expected by society to be demonstrated in social or professional settings.
Q24: Calculate the ΔG°<sub>rxn</sub> using the following information.
Q36: Which of the following represents the integrated
Q62: Dinitrogen tetroxide partially decomposes according to the
Q87: Consider the following reaction at equilibrium.What effect
Q89: Based on the figure above,the boiling point
Q91: Which one of the following salts,when dissolved
Q93: What is the osmotic pressure of a
Q95: Determine the K<sub>b</sub> for CN⁻ at 25°C.The
Q112: In which of the following processes does
Q157: A 100.0 mL sample of 0.10 M