What is the pH of a 0.50 M H2Se solution that has the stepwise dissociation constants Ka1 = 1.3 × 10-4 and 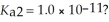
Definitions:
Physician
A professional who practices medicine, which involves promoting, maintaining or restoring health through the study, diagnosis, prognosis, and treatment of disease, injury, and other physical and mental impairments.
Physical Symptoms
Observable or tangible signs of a condition or disease, such as pain, fever, or a rash, in contrast to psychological symptoms.
Conversion Disorder
A mental condition in which a person experiences neurological symptoms, such as blindness or paralysis, without a physical cause, believed to result from psychological stress or conflict.
Blindness
The state or condition of being unable to see due to injury, disease, or a congenital condition.
Q9: What is △n for the following equation
Q24: Determine the rate law and the value
Q63: The gas OF<sub>2</sub> can be produced from
Q68: A 100.0 mL sample of 0.18 M
Q90: Use the tabulated half-cell potentials to calculate
Q106: Determine the molar solubility of Al(OH)<sub>3</sub> in
Q118: Why aren't solids or liquids included in
Q130: What is the hydronium ion concentration and
Q131: A 25.0-mL sample of 0.150 M hydrocyanic
Q152: What is the [CH<sub>3</sub>CO<sub>2</sub>-]/[CH<sub>3</sub>CO<sub>2</sub>H] ratio necessary to