Multiple Choice
Calculate the pH for an aqueous solution of acetic acid that contains 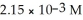 hydronium ion.
hydronium ion.
Definitions:
Related Questions
Q19: Explain how the order of a reaction
Q21: Consider the following reaction at equilibrium.What effect
Q55: Use the standard half-cell potentials listed below
Q55: Determine the partial pressure of oxygen necessary
Q59: Which of the following is an Arrhenius
Q61: Determine the value of K<sub>c</sub> for the
Q68: In an _ solution,red blood cells _.<br>A)
Q72: A galvanic cell consists of a Al<sup>3+</sup>/Al
Q102: The first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+ O<sub>2</sub>(g),has
Q136: Give the equation for a saturated solution