Calculate the pH for an aqueous solution of pyridine that contains 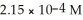 hydroxide ion.
hydroxide ion.
Definitions:
Acetyl CoA
A molecule that plays a key role in metabolism, serving as a substrate for the Krebs cycle and fatty acid synthesis; it links glycolysis and pyruvate oxidation with the citric acid cycle.
Phosphorylation Reactions
Chemical reactions in which a phosphate group is added to a molecule, such as a protein, altering its function.
Exergonic
Describes a chemical reaction that releases energy, typically in the form of heat, to its surroundings.
Hydrolysis
A process where a molecule's bond is severed by incorporating water.
Q2: Identify the change that will always shift
Q2: What volume of a 0.716 M KBr
Q24: Identify the solute with the lowest van't
Q28: Describe the relationship between molecular structure and
Q38: What is the pH at the equivalence
Q62: Identify the solute with the highest van't
Q67: Consider the following reaction: 2 NO(g)+ O<sub>2</sub>(g)⇌
Q92: What data should be plotted to show
Q93: Given the following equation, H<sub>2</sub>O(g)+ CO(g)→ H<sub>2</sub>(g)+
Q113: Identify the indicator that can be used