Calculate the pH of a solution that is 0.210 M in nitrous acid ( 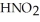 ) and 0.290 M in potassium nitrite (
) and 0.290 M in potassium nitrite ( 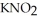 ) .The acid dissociation constant of nitrous acid is 4.50 ×
) .The acid dissociation constant of nitrous acid is 4.50 × 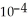 .
.
Definitions:
Behavior-Based
Refers to approaches or interventions grounded in the observation and modification of behavior as the primary method of treating psychological conditions or organizational problems.
Affiliative Leadership
A leadership style focused on creating harmony and building emotional bonds within a team or organization.
Risk Taking
The action of engaging in behaviors or decisions that involve a possibility of negative consequences in the hope of achieving a desired outcome.
Q41: Is the activation energy for a forward
Q46: Which of the following solutions would have
Q76: Which of the following nuclides are most
Q78: K<sub>c</sub> is 1.67 × 10<sup>20</sup> at 25°C
Q84: Determine ΔG°<sub>rxn</sub> using the following information. 2
Q86: Identify the indicator that has two endpoints.<br>A)
Q92: What is the pH of a 0.50
Q92: What is electrolysis?
Q144: Use the standard half-cell potentials listed below
Q152: The stronger the acid,then which of the