Calculate the percent ionization of nitrous acid in a solution that is 0.205 M in nitrous acid 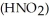 and 0.295 M in potassium nitrite (
and 0.295 M in potassium nitrite ( 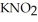 ) .The acid dissociation constant of nitrous acid is
) .The acid dissociation constant of nitrous acid is 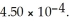
Definitions:
Solar Keratosis
A rough, scaly patch on the skin caused by years of sun exposure, often considered a precancerous growth.
Ritalin
A stimulant medication commonly used to treat attention deficit hyperactivity disorder (ADHD) and certain sleep disorders.
Adverse Effect
An unexpected, bad result; also known as an adverse reaction. The harm a patient experiences by a medication that has been prescribed by a health care professional and taken as instructed; an unexpected reaction to a drug taken for therapeutic purposes.
Administration Route
The path by which a drug, fluid, poison, or other substance is taken into the body, such as orally, intravenously, or topically.
Q2: Give the name of the reaction that
Q14: Which of the following nuclides is most
Q37: Above what temperature does the following reaction
Q46: Calculate the equilibrium constant for the following
Q68: Why are iron nails coated with zinc?
Q71: Which one of the following salts,when dissolved
Q117: Which of the following acids (listed with
Q136: Which of the following metals will not
Q143: What are the units of k in
Q149: What is the pH of a solution