Calculate the pH of a solution that is 0.210 M in nitrous acid ( 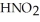 ) and 0.290 M in potassium nitrite (
) and 0.290 M in potassium nitrite ( 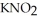 ) .The acid dissociation constant of nitrous acid is 4.50 ×
) .The acid dissociation constant of nitrous acid is 4.50 × 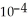 .
.
Definitions:
Desired Level
A specific, pre-determined standard or goal that an individual or organization aims to achieve.
Historical Approach
The method of studying phenomena by examining their development and evolution over time, often using historical records and trends.
Past Performance
Historical results achieved by an individual or organization, used as a benchmark for evaluating current capabilities.
Benchmark
is a standard or point of reference against which things may be compared or assessed.
Q4: Which of the following is a triprotic
Q5: All of the following are examples of
Q12: Define allotrope.
Q50: Which one of the following has the
Q66: Given the following balanced equation,determine the rate
Q67: A 100.0 mL sample of 0.20 M
Q68: Why are iron nails coated with zinc?
Q70: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS,AgCl,NH<sub>4</sub>Cl,and ZnS.Identify the
Q96: Calculate ΔS°<sub>rxn</sub> for the following reaction.The S°
Q143: Determine the pH of a 0.62 M