Calculate the percent ionization of nitrous acid in a solution that is 0.205 M in nitrous acid 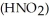 and 0.295 M in potassium nitrite (
and 0.295 M in potassium nitrite ( 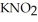 ) .The acid dissociation constant of nitrous acid is
) .The acid dissociation constant of nitrous acid is 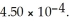
Definitions:
Salespeople
Individuals specializing in the sale and promotion of products or services, often employing various techniques to meet sales targets.
Evaluative Listening
A listening style where the listener analyzes or judges the content of a message, often assessing its truthfulness, usefulness, or relevance.
Emotion-Laden Words
Words specifically chosen to evoke emotional responses from a listener or reader to influence thoughts or actions.
Listening
The active process of receiving and understanding messages communicated by others, often emphasized as a key skill in effective communication and sales.
Q13: Use the tabulated half-cell potentials to calculate
Q17: Which of the following is the strongest
Q23: Calculate the mass defect in Fe-56 if
Q24: Identify the base that is in baking
Q56: Calculate the ΔG°<sub>rxn</sub> using the following information.
Q66: Which of the following is a Lewis
Q68: Give the standard states for a gas,liquid,solid,and
Q99: Give the name of the reaction that
Q124: A 8.0 × 10<sup>-3</sup> M aqueous solution
Q145: Hydrogen iodide decomposes at 800 K via