What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 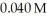 NaCN? Assume that the volumes of the solutions are additive and that Ka =
NaCN? Assume that the volumes of the solutions are additive and that Ka = 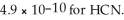
Definitions:
Ordinary Person
An average or typical individual without specialist knowledge or exceptional characteristics.
Negligence
The failure to exercise the care that a reasonably prudent person would in similar circumstances, often leading to harm or damage.
Intended to Harm
Actions or behaviors designed with the purpose of causing physical or emotional injury to others.
Tort
A civil wrong not arising from a breach of contract. A breach of a legal duty that proximately causes harm or injury to another.
Q22: A weak acid is titrated with a
Q29: An aqueous solution of ammonia is found
Q36: Calculate the solubility (in g/L)of silver chromate
Q75: Calculate the cell potential for the following
Q76: Determine the pOH of a 0.188 M
Q119: What is the overall order of the
Q129: Express the equilibrium constant for the following
Q139: What is the difference between average reaction
Q142: A 1.00 L buffer solution is 0.250
Q148: Determine the molar solubility of AgI in