A buffer is prepared by dissolving 70 millimoles of solid NaOH in 500.0 mL of solution with a concentration of 50 mM H2A.Which of the following are NOT true for the buffer?
I After reaction there is 30 mmol HA− and 20 mmol A2− in solution.
II pH is calculated using 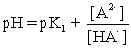 .
.
III After reaction there is 50 mmol HA− and 20 mmol NaOH in solution.
IV pH is calculated using 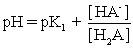 .
.
V pH = 14 - pOH where pOH is calculated from excess OH−.
Definitions:
Osmosis
The movement of water molecules through a semipermeable membrane from a region of lower solute concentration to one of higher concentration, balancing solute concentrations on both sides of the membrane.
Urine pH
A measure of the acidity or alkalinity of urine, which can be an indicator of various health conditions or dietary influences.
Glomerular Membrane
A semi-permeable membrane in the kidney's glomeruli that filters blood to form urine.
Bacterial Toxins
Substances produced by bacteria that can cause disease in humans and animals.
Q10: The equilibrium constants for a diprotic acid
Q11: For the reaction HCN (aq)+ H<sub>2</sub>O (l)<font
Q11: _ is any chemical of interest.<br>A)Analyte<br>B)Species<br>C)Replicate<br>D)Aliquot<br>E)Bulk
Q11: The null hypothesis for the comparison of
Q13: A buffer is prepared from NaH<sub>2</sub>PO<sub>4</sub> and
Q14: _ is a consistent error that can
Q16: All of the following are true for
Q17: In which solution is solubility of silver
Q20: _ assets refer to the accumulated intellectual
Q241: For a college student who wishes to