Calculate the pH for a buffer that is 0.10 M in NaO2CCO2Na and 0.50 M in NaO2CCO2H.K2 = 5.42 × 10−5, 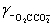 = 0.333,
= 0.333, 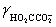 = 0.76
= 0.76
Definitions:
Bank Statement Adjustment
The process of reconciling the difference between a bank's record of an account's balance and the amount shown in the account holder's books.
Company Books Adjustment
The act of making entries or corrections to the financial records of a business to reflect accurate and up-to-date accounting information.
Safe Deposit Box
A secure container, usually found at a bank, where customers can store valuable personal items.
Bank Statement Adjustment
The process of modifying the balance on a bank statement to reflect transactions that have not been recorded by the bank by the end of the reporting period.
Q2: A 100.00 mL solution of copper(II)chloride is
Q3: Homogeneous precipitation occurs when a precipitant is
Q3: When extracting a sample with a liquid,the
Q4: Which statement(s)is(are)defined INCORRECTLY?<br>I Ohmic potential is the
Q6: The end point for a titration can
Q7: Electrolysis of 200 mL of a solution
Q7: The number of plates,N,for a capillary electrophoresis
Q7: Which statement below is INCORRECT for the
Q17: Modern combustion analysis uses dynamic flash combustion.What
Q55: _ refers to an examination of facts