Calculate the pH for a buffer that is 0.10 M in NaO2CCO2Na and 0.50 M in NaO2CCO2H.K2 = 5.42 × 10−5, 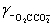 = 0.333,
= 0.333, 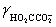 = 0.76
= 0.76
Definitions:
Headcount
The total number of individuals working for a company, either as full-time employees or in positions equivalent to full-time employment.
Hiring Freeze
A temporary policy to cease or significantly slow down the recruitment of new employees, typically implemented to reduce costs.
Efficiency
The ratio of the output to the input of any system, indicating how well resources are utilized.
Measure
A method or standard used for quantifying attributes or performance in order to assess, compare, or track efficiency or progress.
Q3: Which is true when electrochemical cells are
Q15: Which of the following is NOT true
Q17: With respect to the six stages of
Q18: Calculate the pH of a 0.2 M
Q18: Calculate the pH of a 0.001 M
Q38: Which of the following measures of the
Q39: In a market economy,economic activity is guided
Q44: Which of the following perspectives of the
Q49: Which of the following personal leadership competencies
Q241: For a college student who wishes to