Calculate Eo and K for the cell composed of a bromate/bromide half-cell and a chlorate/chlorine dioxide half-cell. 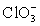 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 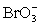 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
Definitions:
Fixed Cost
Expenses that do not change with the level of production or sales over a certain period, such as rent or salaries.
Output
The amount of goods or services produced by a person, machine, or industry.
Marginal Product
Marginal product refers to the additional output generated by employing one more unit of a specific factor of production, holding all other factors constant.
Labor
The exertion of mental or physical strength by humans to create goods and services.
Q5: The advantages of sequential injection over flow
Q10: A(n)_ is a suspension of a solid
Q10: Quinine sulfate in 0.5 M sulfuric acid
Q14: Aqueous lead precipitates when mixed with aqueous
Q15: A 100.0 mL sample of a solution
Q16: All of the following are true for
Q18: Which of the statements below is(are)TRUE regarding
Q43: How are the elements in the balanced
Q62: In the context of the Weber's classification
Q64: How does the eight-step governance process of